Chemistry in Fantasy
“The Blacksmith was right; you can’t make a gold sword.”
“But I have to get her highness a golden sword in three days for the duel! I don’t see how the sword can’t be like any other metal sword that anyone can just whip up!”
Kasper pinched the bridge of his nose. “It’s not that one can’t physically make a gold blade, it’s not practical for battle. Gold is soft, is ridiculously heavy, and is extremely expensive.”
The messenger leaned across the table; Kasper could see the desperation in their eyes. “But I told them I would pay!”
“You don’t understand; you can’t just pay for it, you need the actual physical amount of gold to make the blade. Do you have a couple pounds of gold lying around? And,” Kasper finished polishing the necklace and set it back into its display case. “It’s the worst metal for a blade, as per my other points.”
Were those tears starting to fill the messenger’s eyes? “And you don’t understand that their highness will chop off my head if I can’t bring her the sword by the end of the week!” The messenger burst into sobs. “Please! You’ve got to help me find a smith who is willing to do help me!”
Kasper sighed. “Look, if you get the sword, will you stop soaking my carpet every time you come in?”
The messenger sniffed but nodded.
“I can’t get you a gold sword, but I can make it look like a gold sword. I have the setup in the back. It may take a few tries, as I’ve only ever plated our silver jewelry in the past, but I think I can get a solid layer over a blade. No promises on how it’ll hold up in a fight, but it’ll at least look decent before that. Now, you’ll need to find a clean blade—”
“DONE!” The messenger flew out the door before Kasper could finish. Kasper messaged the back of his neck. He hoped this small favor wouldn’t come back to bite him in the butt once the queen found out. He quite liked his head where it was.
Chemistry and Fantasy (More Like Chemistry in History)
Welcome to the post where we get to dive into a few ways we can incorporate chemistry concepts into fantasy worldbuilding and plot!
Before we begin, I do want to say that this became a much bigger post than expected! I didn’t want to retread old ground, as I have already written two posts on “Science in Fantasy,” just not specifically focusing on chemistry. Therefore, I choose topics that all had to do with art, and I found myself going into the real-life history of chemistry than I was planning. Most of the “cool incorporation ideas” are ones that we can steal straight from history class.
But that makes sense; most fantasy writers don’t incorporate any amount of basic chemistry into their worldbuilding, even when it comes to its crucial role in art. This is because of the false dichotomy where “fantasy” cannot mix with “science” (See my Science in Fantasy post).
And when we look at chemistry in history, especially in the context of art, we can see how integral chemistry is in influencing culture and interactions across the world. Art is a driving force behind many human interactions both in and out of a given culture. In this way, we can steal from the real world setting to spice up our fantasy worlds.
Batteries and Electroplating
Electroplating…
This probably wasn’t the topic you thought of when you saw “chemistry” and “fantasy” together. The idea of using electrochemistry (which is exactly as it sounds: the combination of using electricity in chemistry) in a fantasy setting, even one fairly scarce in magic, feels a bit too anachronistic. It feels like we’re in the wrong genre.
I thought that too, until I recently ran a Dungeons and Dragons session that revolved around the use of electroplating in jewelry production. The more I investigated it, the more I realized that not only could electroplating work in a fantasy setting, but so could batteries.
Before we get into electroplating, we need to talk about the basics of a battery because both the battery and electroplating quite a lot of the same chemistry to work. (And you need a battery to do electroplating, so I need to convince you that the first is possible before I convince you of the latter).
Now, I know what you’re thinking: aren’t batteries way too modern for a fantasy setting?
Firstly, I wouldn’t bother trying to replicate medieval Europe in a magical fantasy setting; most depictions used in fantasy settings are highly inaccurate anyway. Secondly, it’s surprisingly easy to make a battery if one has the right metals (making it portable is another story).
In fact, one can make a sort-of battery by harnessing the power of static electricity using some metal and a glass jar. This would be the Leyden jar, an early predecessor to the battery built in the 1700’s and even used by Benjamin Franklin for experiments. However, the first real battery was created around 1800 using an alternating stack of Zinc and Copper discs, with salt-soaked cloths between each discs This was the Voltaic pile.
Let’s talk about how it works: each zinc-salty cloth-copper set is a voltaic cell. Now, I can’t get into the nitty gritty of the redox reactions, so what you need to know is that at the molecular level, the electrons surrounding metal ions are loosie-goosy. These electrons can move from one metal to the other should the metals be connected by a wire AND dissolved salt in the water or a connecting salt bridge. The energy created from the movement of these electrons is… electricity!
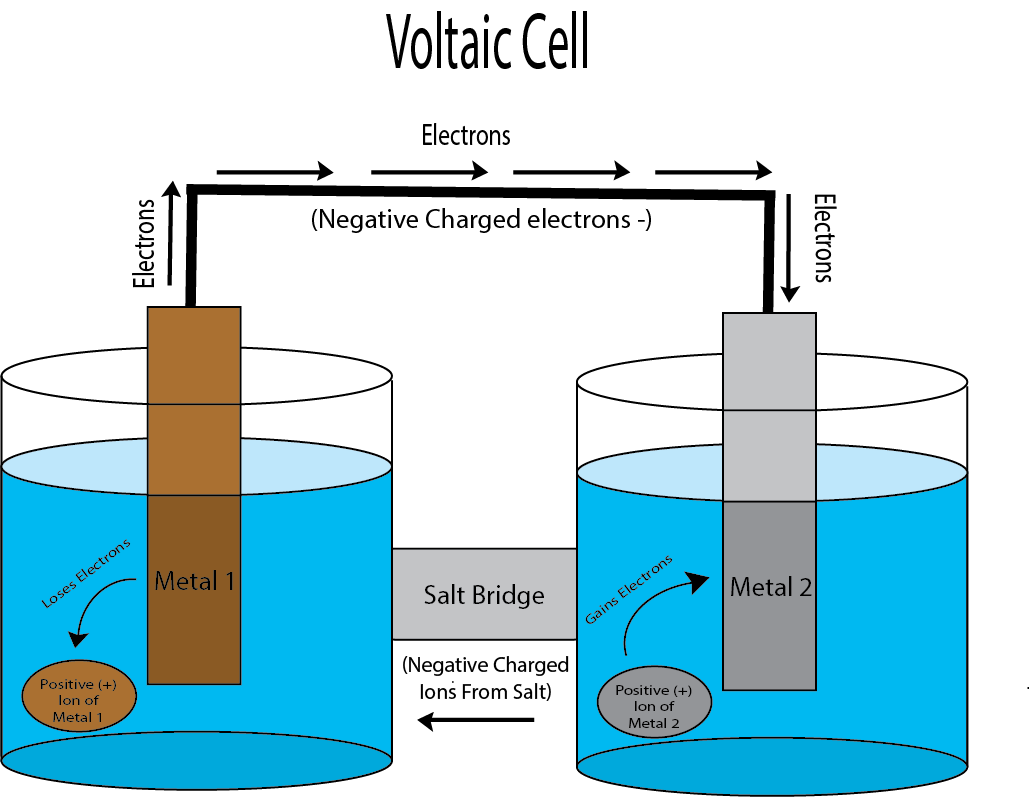
You’ll notice that there are atoms in the image that either hop on or off the metals. These are called “ions,” and they are floating around in the aqueous water. “Ion” is what we call a single molecule with a charge, and is what makes up a lot of salts! (Hence why I keep saying “salt bridge”). The ions are key to allow the reaction to continue once it starts.
The direction the electrons flow in the setup is determined by how well one metal hugs onto electrons compared to the other. In our example, copper is going to “hug” electrons more than zinc, so the electrons will flow from the zinc metal to the copper. The thing is, we also need our positive charges to move around in this setup between the zinc and copper, so that’s where the salt in the water (or the salt-soaked cloth) comes in! Without it, the electrons would move just a bit, and then stop.
TLDR: Metals with a salt-soaked cloth in between make the charges go round, and we can harness those charges for energy! In theory, your fantasy setting can create batteries!
Now you’re probably thinking… that was a lot for a battery. What does that have to do with electroplating?
Well, electroplating uses similar chemistry! Imagine the same setup like the voltaic cell, but unlike the voltaic cell, we won’t just have the electrons naturally move from one metal to the next. We use the energy stored in our makeshift battery to force the electrons to move from one metal to the other!
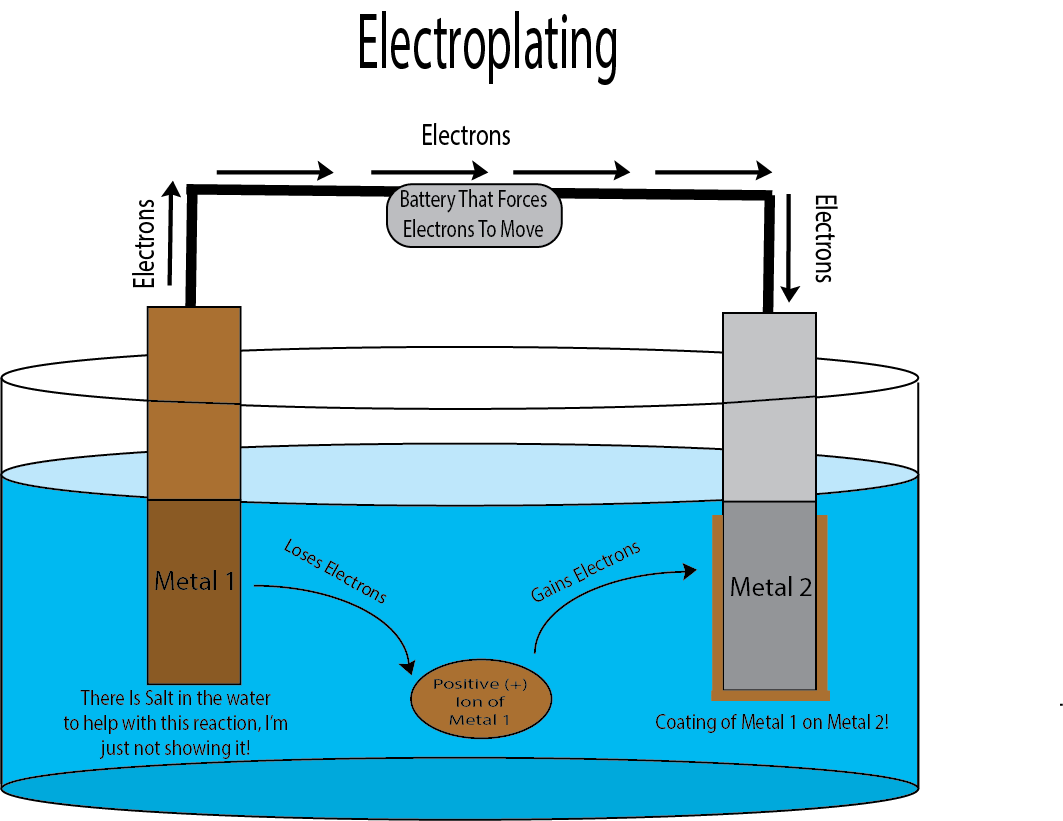
How does the plating happen? Well, you know those metal ions I was mentioning earlier that are floating around in the water? You noticed that the metal that was gaining the electrons also got ions to stick to them? (See image) That’s what’s happening for electroplating, except in this case, metal 1 ions will not stick to metal one. Instead, metal 1 ions will stick and coat the outside of metal 2.
What does this mean for Worldbuilding or Plot?
It means that you can easily have a jeweler who can add silver to the outside of your weapon if you need to fight werewolves. Or, your characters can make a cheap metal look like solid gold if they use electroplating. The characters can also use the reaction to “dissolve” metal into an aqueous solution. Heck, it can be a cool job or hobby one of your characters has!
Spoilers for a one shot (Look away if you think you’ll ever play D&D with me): For one of my recent sessions, I used the chemistry above to put a TON of silver into a water supply. The lycanthropy community in a town was exposed to the tainted silver-water and giving the community rashes and dermatitis. The players had to figure out where and how the silver was getting into the water.
Spoilers done.
But I think the big takeaway here is that your fantasy world can absolutely have batteries, which I think can open up some wild possibilities! Do with that information what you will!
Colored Chemical Explosions, aka Fireworks!
I vaguely remember reading Philip Pullman’s “The Firework-Maker’s Daughter” when I was growing up. A brief online search reminded me that while this book is a fun read, it does not actually base its plot on actual firework making techniques, but on a more mystical world and fairytale plot.
Which is a shame, because fireworks are the coolest example of chemistry as an ancient art that persists even to this day. According to Google, fireworks were developed in China somewhere around the year 1000, producing not only the art of pyrotechnics but also the profession. This isn’t surprising at all, as gunpowder (the key component in fireworks) was invented in China over 100 years earlier.
If you’re keeping track of where this would land in the classic European Middle Ages IRL, this would place the invention of fireworks and gunpowder towards the last quarter of the time period (according to Google). Therefore, the development and use of gunpowder, firearms, and fireworks would absolutely exist in worlds inspired by the “Dark Ages.”
So why fireworks?
Because they’re awesome!

Remember our discussion up above about the electrons in metals? That’s coming into play here!
When a firework explodes, the metals are exposed to heat from the fuel (gunpowder) in the presence of an oxidizer (a compound that contains oxygen necessary for the fuel to burn). This is all held together by a binder of some sort, usually a starch. (This site contains the exact image that we used back at a museum I volunteered at to explain this process. Check it out!)
That heat is energy, and that energy is taken up by the electrons of the metal atoms. This excites the electrons, which raises its energy levels to an excited state. However, this electron can’t stay excited for long, and therefore drop back to resting levels. This happens over and over again as the electrons are exposed to heat. When heading back to resting levels every time, the electrons release that energy in the form of light, which has a unique color depending on the given metal! That’s how we get our sprays of color during a firework’s display!
What Does This Mean For Worldbuilding or Plot?
Sweet! So after that crash course on firework chemistry, let’s talk use. A character may be on a quest to find the perfect chemical formula to create the most spectacular firework. Maybe their hometown doesn’t have access to certain metallic salts and they head out to find their own stash for their art.
Or, maybe there’s a new metal in your fantasy world that is horded by, say, a dragon, and your firework maker desperately wants to experiment with this new metal. Would they bargain with the dragon? Would they try to steal this metal?
Your fantasy chemist can also use this same chemistry to do something called a flame test, which can help characters determine what metals are in a mysterious compound (provided said compound isn’t dangerously flammable). Could come in handy depending on the situation!
What if your fire worker is kidnapped and is forced to produce firearms for the antagonist? After all, even the most primitive fireworks are essentially rockets.
Or, what if you’re in a Mistborn-like world where “burning” metals produces magic, but you must literally burn the metals, and the system works using sparklers and firework chemistry? (We’re going a little off of chemistry here, but wouldn’t that be cool?)
And that’s just for character building; adding “firework maker” as a scientific and artistic profession in a fantasy setting would provide such a cool flare (pun intended) to the worldbuilding. Who studies fireworks, how prestigious the job is, and when fireworks are used are fascinating worldbuilding questions.
Move aside, potion-making and animatronic alchemists: the colorful pyrotechnic is in style!
The Art of Colored Chemistry

Much of this comes from the beginning of Adam Roger’s book, “Full Spectrum: how the science of color made us modern.” I highly recommend it!
The creation of pigmented paint is surprisingly scientific, especially for the color of white. Some of the earliest pain was made by grinding up seashells, egg shells, or anything that would have calcium carbonate. Ancient civilizations like Egypt and Rome used the toxic lead white, which was, unsurprisingly, dangerous. Eventually, Aguste Rossi would accidentally discover the non-toxic white of titanium dioxide during the Industrial Revolution.
People all throughout human history have experimented with ways to capture color for art. One cannot just grind up anything in nature and have it be usable permanent paint. Grind up chalk, wet it, and paint it on any given surface, and I can guarantee you that it’s not going to stick around once it dries (pun intended). According to Roger, the earliest paints were of ground up rock fragments with oxidized metals (like rust; that’s iron oxide), mixed with clay or sand: this mixture was called an ochre. This gave the earliest paints the colors red, yellow, orange, and purple (and I am assuming brown, although Roger’s doesn’t say). Black may have been from carbon, like leftover charcoal.
Roger’s also notes that red ochre plus resin made a solid glue (quick fun fact).
We can’t go through the every single pigment, but we can look at a few! The first synthetic pigment is allegedly the combination of a metal containing copper (azurite or malachite), silica, limestone, and a base to create Egyptian blue. Then, you heat up the mixture to 800-1000 degrees Celsius according to the Wikipedia article to get that amazing pigment. Another blue pigment, Maya blue, probably came from combining anil leaves and montmorillonite clay and is highly resilient to both time and acid exposure.
Now, remember how I said that you can’t simply grind up anything to make a paint? That’s because paints need something to make them bind, and a solvent to dissolve the pigment (which can be just water). Some ancient painters mixed animal fat into their prepared pigments. Ancient Egyptians used eggs, while Renaissance painters took the oil from things like walnuts to produce the famous oil paints.
We can even go further and look at the art of painting on cloth or canvas; dyeing. As far back as the 13th century, the use of Tyrian purple (a pigment made from Murex snail mucus) can be documented. Tekhelet was a highly sought after for prayer garments, and the pigment was likely isolated from the Hexaplex Trunculus snail glands. The first synthetically created dye, the bright purple-ish Mauveine, was discovered (once again by accident) by the teenage William Perkin by mixing aniline (yellow), p-toluidine (colorless), o-toluidine (colorless and toxic), and potassium dichromate (dark orange) in sulfuric acid. He was trying to recreate a malaria medication, but instead revolutionalized the dye industry.
What Does This Mean for Worldbuilding or Plot?
Why would I spend so much time on how chemistry is incorporated into paint? What if I told you that the technology available to different civilizations to make color drove a significant portion of trade throughout real life history? The location of one’s civilization is determines what resources that culture can use to produce art and, by extension, the style of said art. The more difficult the chemistry needed to make the art supplies, the more rare/expensive that supply will be.
Why this is important is that art and art techniques drove a lot of trade in human history, and it should in a fantasy setting. The maritime Silk Road, according to Roger’s book, brought the trade of colored ceramics across the continent (it implies painting techniques did as well, although he doesn’t explicitly say it). Too often do we have fantasy books that have trade routes that only focus on necessities that, while important, are only part of the trade in the world. People will pay for art and color. People will pay for unnecessary, but extremely nice, luxuries.
Because of this, the chemistry of color should be extremely valuable.
And valuable resources can absolutely be used to great effect in a fantasy story; that’s always a great motivator!
You can have chemists trying to reproduce the paint they saw on one of their travels. You could have a chemist accidentally create the next big dye or paint color like William Perkin that allows a previously expensive color to be readily available. Said chemist may or may not like the attention and could have people try to steal their accidental discovery. Maybe we’re in the point of view of someone struggling to make a colored paint because a binding agent isn’t working well with their chosen pigment or canvas, and that starts them down a series of experiments.
Maybe you have a town that is famous for their paints and/or dyes and everyone wears striking colors. Or, we could take a tip from history and make certain colors display wealth because of how hard it can be to make a particular pigment in the setting. Maybe a group protects the secret to making certain pigments that hold their color loner and are more resilient to weather erosion.
These ideas may not necessarily drive the entire plot of a story, but it could provide some amazing side quests or personal character motivations.
Takeaways
I’ll keep this brief since each section had its own sort of “takeaway” already!
We can always look to history for chemical innovations for our non-modern fantasy worlds. I don’t think people tend to realize that chemistry is an integral part of many civilizations, even if they didn’t outright call it “chemistry.” And I love being able to show that the sciences and arts are more integral than most people may realize.
And I could have chosen more topics! Glass making, metal work, concrete production, cosmetics, soaps, brewing, etc. I’m not joking: I could write a section on soap making! And I only touched on it in the firework section, but the invention of gunpowder is one of the coolest things ever (in my opinion).
And we may have another post to touch on these topics! This was too much fun to not do again!
